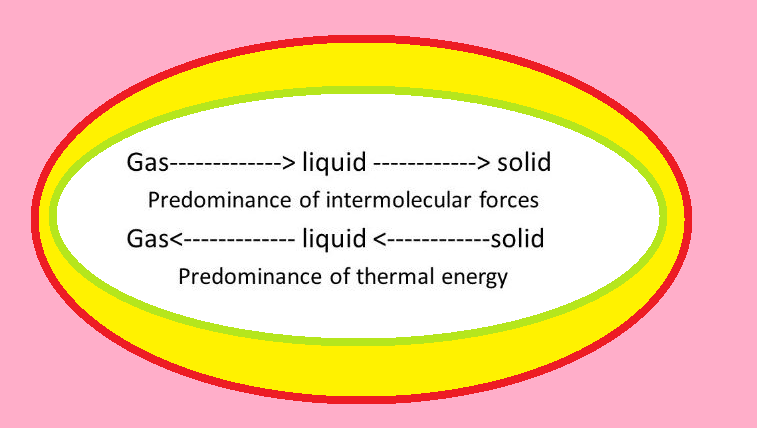
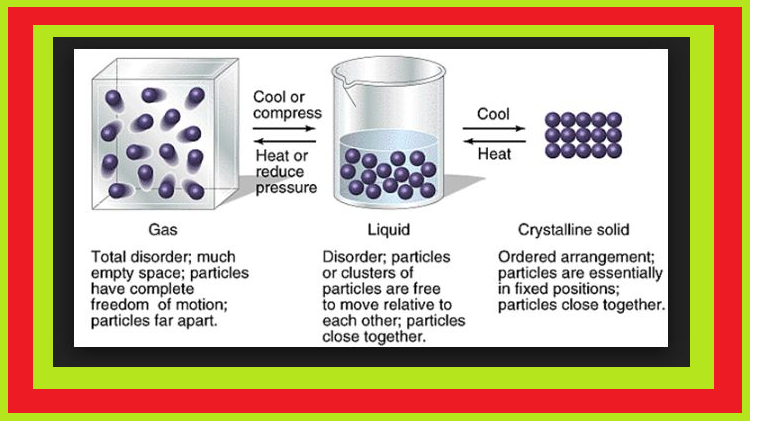
Thermal Energy :
`text(Definition :)` Thermal energy is the energy of a body arising from motion of its atoms or molecules.
● It is directly proportional to the temperature of the substance.
● It is the measure of average kinetic energy of the particles of the matter and is thus responsible for movement of particles.
● This movement of particles is called thermal motion.
● It is directly proportional to the temperature of the substance.
● It is the measure of average kinetic energy of the particles of the matter and is thus responsible for movement of particles.
● This movement of particles is called thermal motion.